March 30, 2020
An innovative lab-on-a-particle approach offers the precision of droplet microfluidics using standard laboratory equipment only. Researcher Joe de Rutte anticipates new opportunities for life sciences collaboration as this breakthrough allows researchers to more rapidly develop new biotechnology products. This work earned de Rutte the SLAS Innovation Award at SLAS2020 (San Diego, CA, USA).

Three years ago, Joseph de Rutte, M.E., a fourth-year bioengineering Ph.D. candidate at the University of California, Los Angeles (UCLA, Los Angeles, USA) launched what he calls a ‘shoot for the moon’ project that left him excited and a bit uncertain. His team set one rule to answer one question: Without using any special devices, could they take particles and make uniform nanoliter-scale droplets?
Droplet microfluidics offers a powerful means to conduct single-cell high-throughput screening (HTS) by using small volume compartments to accumulate high levels of secreted factors or other molecules released from cells for accurate detection. In spite of this potential, however, the necessity of specialized equipment limits widespread adoption by end-users.
“We worked with the idea that we could make microparticle technologies to conduct complex assays using existing lab infrastructure – items as simple as well plates and pipettes. At the same time, we wanted our new droplet technology to be compatible with more complex equipment that might be in place, such as flow cytometers,” explains de Rutte, who works in the lab of SLAS member Dino Di Carlo, Ph.D., vice chair of the Department of Bioengineering at UCLA and a scientific advisor on the editorial board for SLAS Technology.
The discovery that emerged from de Rutte’s research – the particle-templated droplet that he calls the dropicle – is an efficient method to analyze and sort cells based on secretions, such as antibodies, cytokines, proteases or other enzymes, that helps expand understanding of cellular heterogeneity fundamental to biology and opens the door to create new biotechnology products, such as biologics and cell therapies. The technology also could aid in the rapid development of new neutralizing antibodies or vaccines – a relevant contribution in the quest to abate the COVID-19 pandemic. The easy-to-distribute technology offers widespread capabilities to researchers.
The breakthrough innovation holds the potential to dramatically extend the reach of single-cell screening technology, as explained in de Rutte’s award-winning presentation, “High-Throughput Encapsulation and Selection of Cells Based on Antibody Secretion Using Lab-on-a-Particle Technology.”
“Work in Dino’s lab is creative and serendipity driven – it’s about noticing interesting phenomenon and using it to the advantage of research,” says de Rutte, whose research launches from the same lab in which previous SLAS Innovation Award Winner Elodie Sollier-Christen, Ph.D., worked. “I had no idea if we could get the dropicle research to work, but I knew that if we could it would be a powerful tool.”
Figuring out a simple process took a surprising amount of effort, he reports. “Some methods completely failed. I can see now that there was no way some of those were going to work, but I also see that each one got us one step closer to success. Sometimes the failures are where you learn the most,” de Rutte says, adding with a laugh: “Maybe it’s my obsessive nature, but I kept hammering away at it!”
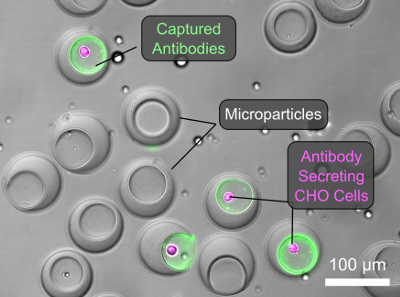
Three years later, after spending a year in the lab without any serious breaks, de Rutte’s obsessive work paid off in the simple process he presented at SLAS2020. To create the lab-on-a-particle, he forms prefabricated, cavity-containing microparticles and loads them into a well plate. The particles’ morphology causes them to settle upright into the well plate with their cavities exposed, ready to be seeded with cells that adhere to the particle matrix via integrin binding peptides. Then de Rutte transfers and agitates the particles and associated cells by pipetting oil and surfactant to generate monodisperse ”dropicles” that encapsulate single cells. After incubating the particles and cells to accumulate secretions and transferring them back to buffer for labelling, the particles and cells can be sorted using high-throughput commercial flow cytometers.
In his research, de Rutte observes that seeded cells fill the cavities of the particles according to single-poisson statistics (in contrast to typical double-poisson statistics for single-cell, single-particle pairs in drops). After dropicle formation, cells maintain high viability over 24 hours. Initial tests with anti-IL-8 producing CHO cells demonstrate the ability to capture and label secretions on particles containing cells without crosstalk to neighboring particles and to isolate cells associated with high anti-IL-8 signal in high-throughput using commercial flow cytometry systems.
Since dropicles form simultaneously, compartmentalization is rapid and can be easily scaled to accommodate large population screens. Further, the associated particle enables additional functionality such as physicochemical cues or cell specific capture antibodies to select out specific cell types. The results demonstrate new capabilities for lab-on-a-particle technologies that can accelerate automation of single-cell assays.
The real key feature is the simplicity of the approach. “When you work in a microfluidics lab it’s easy to say, ‘Oh we could use this!’ Instead, we needed to develop this technology so that anyone could use it in a basic biology lab from the beginning to end of the assay,” says de Rutte.
Another advantage of his innovation is the compartment aspect. “Because we were making the compartments with microparticles, each one offers us a solid surface with which to interact,” he explains. “In typical microfluidic droplet work, a cell inside of a droplet just floats around. In this technique, the solid surface gives researchers an opportunity to perform a chemical reaction on a surface with a small capture bead, for example.” The surface also offers researchers opportunities to have a site for accumulating signal or conduct bar coding or other technical activities that are difficult to do with other microfluidic approaches.
“The original concept was something that Dino kindled over a few years,” de Rutte says. “What he and others began to notice is that when you develop microfluidic technology, and you want to have others use it or you want to commercialize it, you have to develop an all-encompassing machine. You need chips, pumps, a read-out – a lot of additional infrastructure. If you make a system where all the researcher has to do is press a button, the technology development generally becomes slower and significantly more expensive. It’s not a simple chip that you can give someone to use.”
In the beginning, because of the fledgling idea’s simplicity and newness, the team had difficulty convincing people of its merits. “When we first started the research, people wanted to know what’s the killer application? We told them about the future potential. When you are applying for grants or submitting papers, people want to see the potential immediately,” says de Rutte, who initially established a five-year timeline to develop the idea. “Thanks to the dropicle’s simplicity, we reached our goals in three years. We can show assays with better results than other microfluidic platforms.”
Visualizing the new technology’s role in life sciences research became clear for de Rutte while attending SLAS2020. “The first thing that clicked for me was just how many people are still using the 96-well plate and automated pipetting robots. It’s a more traditional route to automation that is still at the fundamental core of the SLAS audience,” says de Rutte, who earned an SLAS Tony B. Academic Travel Award. de Rutte was among 53 students, graduate students, post-doc researchers and junior faculty members from 12 countries who received awards, which recognize up-and-coming researchers who have demonstrated outstanding achievement in laboratory science and technology. The award covers travel costs, including conference registration, airfare and hotel accommodations, to enable these scientists to present research.
“Going to SLAS2020, seeing the interest and receiving the award drove home that we created something useful that has the potential to transform research,” says de Rutte. “The applications for this technology are very current, from the interest in cell secretions and CAR T-cell therapies, to understanding cell heterogeneity.”

Whether it’s giving a prize-winning presentation, pitching new business to potential investors or explaining his innovation to possible collaborators, de Rutte says what matters is conveying science and technology in a simple manner.
“Our lab spends a good amount of time developing how we convey ideas,” says de Rutte. “When you’re presenting to people from completely different fields, a lot of field-specific jargon becomes difficult to understand. Even if you have to simplify a technical explanation, it’s much more beneficial to convey that information so that people can grasp more from the presentation.”
He likes that the SLAS Innovation Award emphasizes the importance of presentation. Knowing that his talk would be to an elite audience, alongside professors with more experience and years of research from which they could draw, de Rutte carefully prepared. “If you can’t explain your science, it makes it much more difficult to collaborate,” says de Rutte, who has an opportunity to submit a scientific manuscript about the research for peer-reviewed publication in SLAS Discovery or SLAS Technology, the scientific journals of SLAS. “I want people to grasp concepts so that technology is adopted and applied in new ways.”
It was his desire to jump into new challenges and problem solving that led de Rutte to change lanes from mechanical engineering to microfluidics and bioengineering after completing his bachelor’s degree at the University of California, Santa Barbara (Santa Barbara, CA, USA). He got a head start on life sciences research when SLAS member Sumita Pennathur, Ph.D., invited him to join her lab for graduate studies at UC Santa Barbara. Her work focuses on understanding and exploiting fundamental micro- and nanoscale phenomenon and coupled physics at the solid-liquid interface to build real-world applications in medical diagnostics, therapeutics and energy.
“I really enjoyed the environment,” de Rutte comments. “Sumita is a great mentor, and at the time I joined her she was working with nanofluidics and electroosmotic flow, very technical work that was fundamental to my growth as a researcher.”
A few brief internships in industry and a four-month stay at the University of Tokyo (Tokyo, Japan) in the microfluidics lab of Shoji Takeuchi, Ph.D., also helped solidify de Rutte’s decision to pursue research. “One of the focuses in the Takeuchi lab is incorporating creativity into research to develop technologies,” says de Rutte, explaining that Takeuchi, who also originally trained as a mechanical engineer, explores hydrogel and biomechanical research.
“It was interesting to me how people with mechanical engineering and chemistry backgrounds could work together on these applications. That helped convince me to take the plunge into a new field,” says de Rutte, acknowledging that his background is unusual for what he does now in working with cells and polymer chemistry.

“When you bring your knowledge from one area and apply it to another, you’re able to see new applications or areas that fall between fields,” de Rutte continues. “Personally, I like to go through a problem in a very methodical way, but also to think about it from a physics perspective. When you have a good grasp of how things work, it makes going through the logics of problem solving easier.”
The UCLA team is excited about the future problem-solving capacity of this technology and how easy it is to share with people. “We’re already starting to work with collaborators around the world,” says de Rutte, who reports that his team is presently exploring ways to commercialize the technology using funds from the SLAS Innovation Award’s $10,000 cash prize to jump start the process.
“It’s simple to send these particles over as a reagent for people to do assays,” says de Rutte. “We’re ready to collaborate and see how others might adopt this technology in ways we haven’t begun to think about. We’re very excited about that aspect.”
Hear de Rutte on SLAS New Matter: Inside the Minds of SLAS Scientists
Learn More about the SLAS Innovation Award and 2020 Finalists!
From SLAS Technology: Droplet Combinations: A Scalable Microfluidic Platform for Biochemical Assays
An SLAS Discovery Review Article: Three-Dimensional Cell Cultures in Drug Discovery and Development